Your Immune System Is Smarter Than Any Drug—If You Know How to Train It
Insights and guidance from Parker Institute for Cancer Immunotherapy's CEO, Dr. Karen Knudsen
Nine cancer patients were scheduled for surgery. Standard protocol: cut out the tumor, hope you got it all, deal with the aftermath.
While they waited for their surgery dates, researchers tried something. They gave these patients a cancer vaccine to see if they could shrink the tumors first.
Some of those tumors shrank so much that the patients no longer needed surgery at all.
Dr. Karen Knudsen, CEO of the Parker Institute for Cancer Immunotherapy, told me this represents a new paradigm in oncology: cure without cutting.
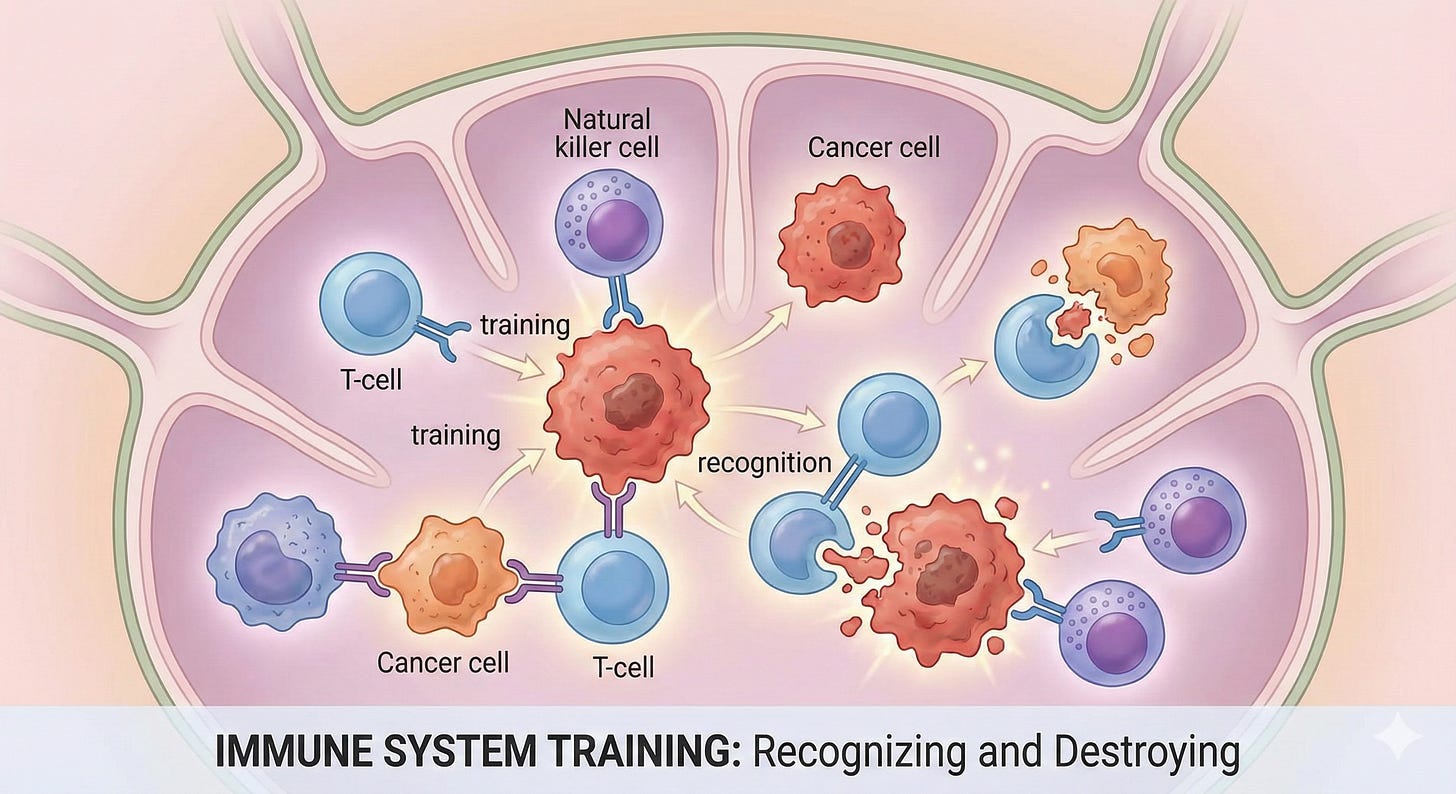
This is what’s possible when you train your immune system to do what it already knows how to do—recognize and destroy cancer cells. Your immune system is doing this right now, as you read this. It’s happening in me, in you, constantly. The question is how we help it do that job better when cancer gets past its defenses.
Immunotherapy was part of my treatment protocol. It’s part of why I’m here. And the American Cancer Society just announced that since 1983, the 5-year survival rate for cancer has risen from 50% to 70%—immunotherapy is a significant driver of that gain.
But here’s what Dr. Knudsen and I lamented:
98% of cancer patients never access clinical trials where the most advanced immunotherapy treatments are available. Most aren’t even offered the option.
Not because the trials don’t exist. Because the infrastructure to deliver them is broken.
Let me show you what’s actually happening with immunotherapy, why most patients can’t access it, and what’s finally changing.
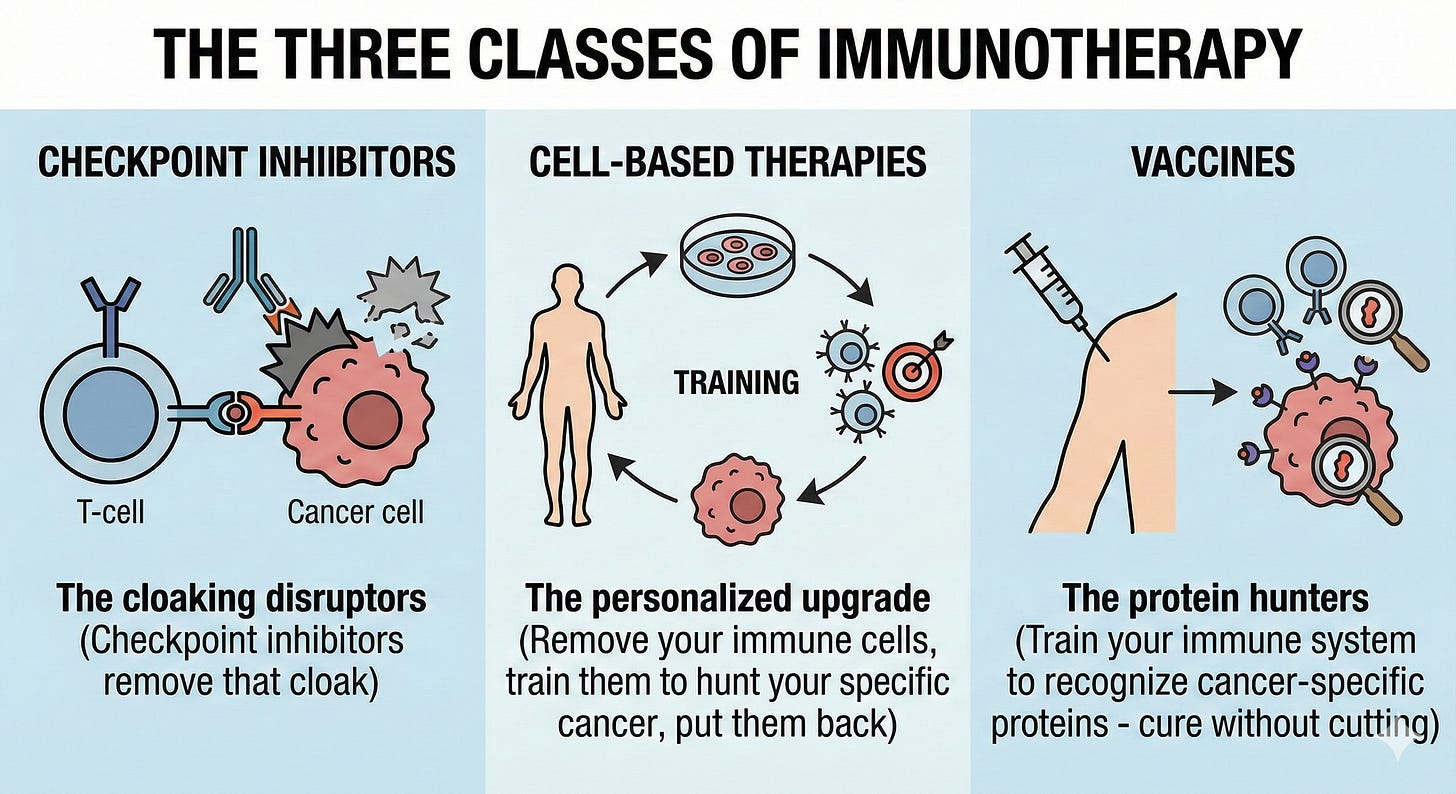
The Three Classes of Immunotherapy
Checkpoint Inhibitors - The cloaking disruptors
Your immune system already sees cancer cells as threats
Tumors use a cloaking mechanism to hide
Checkpoint inhibitors remove that cloak
Now used for melanoma, lung cancer, and bladder cancer
Moving earlier in the disease course (a sign they’re working)
Cell-Based Therapies - The personalized upgrade
Remove your immune cells, train them to hunt your specific cancer, and put them back
A single dose can cure
Working incredibly well for blood cancers (leukemias, lymphomas, multiple myeloma)
The race: how to scale this to solid tumors (breast, prostate, colorectal)
“Multiple myeloma went from being one of the most difficult cancers to treat to now having so many options; we need to think about quality of life in deciding which one to go for.”
Vaccines - The protein hunters
Train your immune system to recognize cancer-specific proteins
Unlike checkpoint inhibitors or cell therapy, vaccines use proven vaccination strategies
Your immune system won’t forget—protection can be for life
Early days but there was a 9-patient study where tumors shrank so much that surgery wasn’t needed
“Cure without cutting” as a new paradigm
“Your immune system once trained appropriately is there beyond just that initial time. It’s with you. It still remains your immune system. It’s the new immune system for us that’s now trained to see these cancers.”
The Clinical Trial Access Crisis
Fewer than 10% of cancer patients participate in clinical trials. The average cancer patient sees 32 different providers during treatment—imagine trying to make 32 hair salon appointments, and you get a sense of the coordination nightmare.
But the access problem isn’t about patient confusion. It’s about infrastructure.
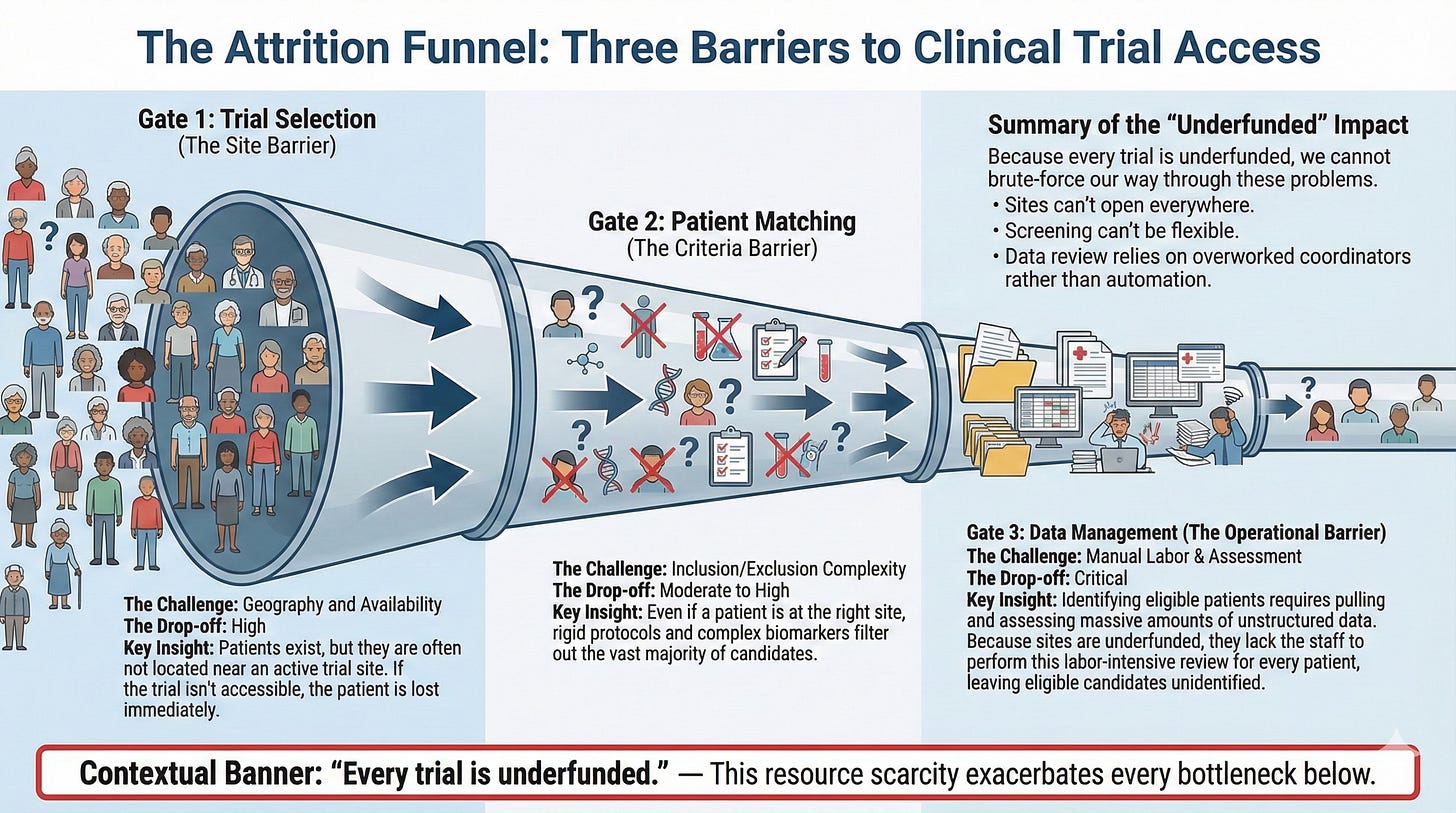
The Three Bottlenecks:
Trial Selection - Should we open this trial at our site? Do we have the right patient population? Are we competing with another trial that would cannibalize enrollment?
Patient Matching - Every trial has inclusion/exclusion criteria. Must have X stage, must have been on Y prior therapies, can’t have ever been on Z drug, must have specific lab values. The list is long. The manual work of checking each patient against each criterion is crushing.
Data Management - Patients in trials get extraordinarily attentive care. Every sniffle, itch, rash gets documented. Pulling down and assessing this data for every patient is labor-intensive.
Every clinical trial is underfunded. Academic centers—where people work specifically because they believe trials are core to cancer care—still can’t hire the teams needed to do this work well.
Community hospitals? Forget it. They don’t have the resources.
So breakthrough treatments sit in academic centers while patients in rural America never get access.
The AI Solution:
Companies like Paradigm Health use Agent AI to solve all three bottlenecks at once
Community hospitals can open trials without massive hiring
First-time rural patients can access cutting-edge care without traveling hundreds of miles
For the first time, they don’t need to hire a whole suite of people. They need to hire some, but not nearly as many.
While we’re talking about access to existing trials, there’s a deeper problem: breakthrough therapies are piling up in laboratories with no path to patients.
The disconnect:
Researchers develop promising strategies for prevention, early detection, and treatment
They prove these work in preclinical models (lab studies, animal studies, human tissue models)
Then they hit a wall
Venture capital used to fund early-stage clinical trials. Not anymore. Now VCs say: “Show me it’s de-risked. Give me a clinical signal first.”
But getting a clinical signal requires running a clinical trial. Which requires funding.
Catch-22.
Dr. Knudsen has been in oncology her entire career—as a scientist, healthcare executive, CEO of the American Cancer Society, and now CEO of the Parker Institute. She’s spent that entire career trying to get more patients access to clinical trials.
The Parker Institute exists to break this cycle.
Their Model:
Only fund high-risk, ambitious science aimed at cures (nothing less)
Doesn’t fund and forget—embeds staff in tech transfer offices
Sees discoveries across all 163 funded scientists
Connects dots: “This plus this equals 12”
Creates patents and companies
Non-profit gives birth to for-profits
17 companies in portfolio, $4B+ raised by those companies
When companies or licensed technologies succeed, the returns fund more science
Everyone wants science to be de-risked, which means not just a great idea in the lab, but show me a clinical signal. That’s where we come in.
The Parker Institute has become the bridge VCs won’t fund. They take great science from labs, get it to clinical signal, then VCs join the syndicate.
Risk-Adjusted Screening
While we’re waiting for breakthrough treatments to reach patients, Dr. Knudsen brought up something practical we can do right now: understand our actual cancer risk.
She runs an experiment. When she’s in a room with thousands of people, she asks how many are “average risk” for cancer. Almost no one raises their hand.
Because they don’t know what that means.
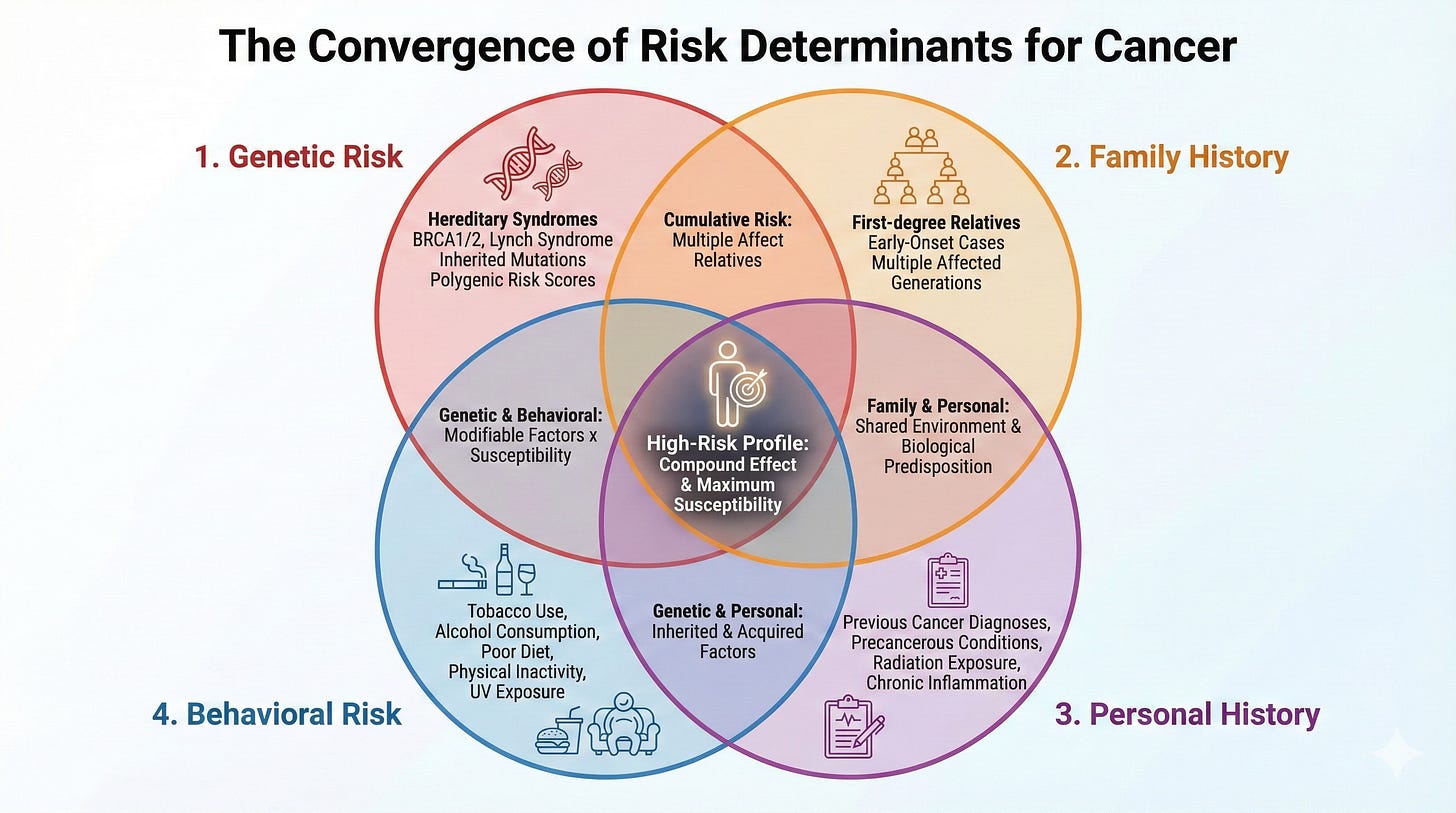
Here’s what actually determines risk:
I’m very grateful that the group where I spit in the tube is going to contact me if the genetic information about me now we know more about, and there is a change in cancer risk.
1. Genetic Risk - Spit in tube, test once, get updates as science evolves
Not all BRCA mutations are equal (I didn’t know this, even as a BRCA1 carrier)
Some are “variants of unknown significance”—we haven’t studied enough people yet
As science advances, these get reclassified
2. Family History - Should be documented annually at primary care
Some cultures don’t discuss what killed relatives (bringing the “evil eye”)
But doctors need this information to adjust screening
Tools exist to help primary care assess if family history changes screening needs
3. Personal History - Past exposures that increase risk
Dr. Knudsen’s radon example shook me - Radon is the #2 cause of lung cancer after smoking. Most people don’t know if they’ve been exposed. It comes through basements, wells, becomes aerosolized when you shower. If you live in a house with a basement or well and haven’t tested for radon, you don’t know your lung cancer risk.
Screening guidelines only cover heavy smokers—but what if you’ve breathed radon for years?
4. Behavioral Risk - 40% of cancers tied to modifiable risk factors
If you know you’re high risk, you can modify behavior
The goal: Move from broad categories (”average risk women should get mammograms at 40”) to individualized screening based on YOUR genetic, familial, personal, and behavioral risk. Dr. Laura Esserman’s WISDOM study paper shows “an individualized approach to breast cancer screening that assesses patients’ risk, rather than automatically giving annual mammograms, can lower the chance of more advanced cancers, while still safely matching people to the amount of screening they need.”
The Blood Test Question
Dr. Knudsen was careful here. Multi-cancer early detection tests (MSEDs) are generating a lot of buzz and a lot of money.
Can they see cancer DNA in circulation? Yes. Same technology used for prenatal genetic testing.
Do they provide clinical benefit? The jury’s still out.
The problem: What do you do when a blood test says you might have breast cancer but your mammogram shows nothing? Does circulating cancer DNA always mean a clinically meaningful tumor?
Your immune system destroys cancer cells all the time. Just because cancer DNA showed up in your bloodstream once doesn’t mean you have a tumor that will kill you.
Clinical trials are running now to answer this question. Where these tests may help first: monitoring for recurrence in patients who had “curative” treatment.
Dr. Knudsen’s advice: Wait for the clinical benefit data before paying out of pocket.
The Patient Agent Solution
Back to that 32-provider problem.
32 providers means 32 different appointment systems, 32 different communication styles, 32 different electronic health records that don’t talk to each other.
The patient is the only person who sees the whole picture. And the patient is also the person going through chemotherapy, radiation, surgery, recovering from all of it, trying to work, trying to maintain relationships.
Current patient navigation supports logistics: appointments, rides, and resource finding. Important, but not enough.
The gap is understanding. What’s happening to me? Why this treatment? What should I expect? What are the side effects I’m experiencing, normal or dangerous?
Dr. Knudsen co-founded Patient Agent (still in stealth mode, piloting with health systems) to solve this.
How it works:
Connects to patient’s own health record
Explains cancer information, treatment plans, and what to expect on different therapeutics
Answers questions about the individual treatment plan
Generates notes TO the care team before visits: “Here’s what we’ve seen in the last four weeks. Here are our questions and concerns.”
When the door opens for your oncology visit, your doctor already knows what you’ve been experiencing
The reverse of current system, where doctor writes notes for the patient
Quote: “Much like the physician writes notes for the patient when they leave, the reverse can also be true of the patient and their caregiver generating a note.”
They’re piloting with patients, caregivers, oncology teams, social workers, and navigators.
The belief: Educating patients and giving them a 24/7 agent acting on their behalf improves outcomes.
What You Can Actually Do
Dr. Knudsen’s advice for anyone facing cancer:
Ask questions. Every single time you’re in the oncologist’s office: Why this therapy? What are my alternatives? What else can I expect?
Take ownership. Don’t apologize for being informed or for pushing back. The best care teams revel in patients who think through what’s right for them.
Be a relentless seeker of information. Medicine is an art. It’s not just about the guideline that says, “here’s the next right thing.” It’s about treating the person sitting across from the oncologist.
My addition: Track your side effects. I created spreadsheets for this because you will not remember what happened three weeks ago when you show up at the doctor’s office. Even if your doctor knows to ask, it won’t be a meaningful conversation without data.
The gap between breakthrough science and patient access is closing. AI isn’t hype in this context—it’s infrastructure. Infrastructure that’s bringing clinical trials to community hospitals, connecting 163 scientists’ discoveries to create companies, helping patients coordinate 32 providers, and understand complex treatment plans.
That infrastructure could save your life. Or the life of someone you love.
From 50% to 70% survival since 1983. Immunotherapy is part of that jump. And we’re just getting started.