Why Your Oncologist May Not Talk About Insulin
And What the Science Actually Shows
Here’s something that should make you angry: The connection between insulin and cancer growth has been studied in major journals for decades, yet most oncologists don’t test for it and weren’t trained to look for it. This is just like the link between exercise and cancer, which was clear in the sports medicine journals but nowhere in oncology.
Dr. Mary Ann Martin sat across from me and said something that stopped me cold: “I was never taught the connection between how cancer can grow in an environment where insulin is left unchecked.” She’s an endocrinologist. This is supposed to be her territory. If she wasn’t taught this connection, what chance does your primary care doctor have?
But the research is unambiguous. Let’s look at what we actually know.
The Science Behind Insulin as Tumor Fuel
When Dr. Martin describes insulin as “fertilizer” for cancer - use a little, great; use too much, dangerous weeds start growing - she’s simplifying a well-established biological mechanism. Insulin doesn’t just shuttle glucose into cells. It activates growth pathways.
Insulin-like growth factor 1 (IGF-1) and insulin share receptors and signaling pathways that promote cell proliferation and suppress apoptosis - the natural cell death process that keeps tumors from forming. When insulin levels stay elevated, these growth signals stay on. A pooled analysis of 17 prospective studies published in The Lancet Oncology found that higher IGF-1 levels were associated with increased breast cancer risk. A 2020 Mendelian randomization study in Cancer Medicine established a causal relationship between elevated IGF-1 and colorectal cancer, with an 8% increased risk per standard deviation of genetically predicted IGF-1 levels.
But here’s what connects this directly to your metabolic health: A large population-based study using NHANES data found that people with hyperinsulinemia (fasting insulin of 10 or higher) had double the cancer mortality risk - a hazard ratio of 2.04 - compared to those without elevated insulin. This held true even in non-obese participants.
The Cremona Study, following over 2,000 people for 15 years, found that those in the highest quintile of serum insulin had a 62% higher risk of cancer death - and a 161% higher risk of gastrointestinal cancer death specifically. These associations were independent of diabetes, obesity, and metabolic syndrome.
This isn’t a marginal effect. This is a meaningful risk that we could be measuring and managing, but largely aren’t.
Why This Isn’t Standard Practice
The insulin-cancer connection lives in the same strange limbo as the exercise-cancer connection: deeply proven, extensively studied - but not in the journals oncologists read. The research appears in endocrinology publications, metabolism journals, nutrition science. It’s robust. It’s replicated. And it’s largely invisible to the specialists treating your cancer.
This isn’t about oncologists being dismissive or uninformed. It’s about how medical knowledge stays siloed. Your oncologist reads the Journal of Clinical Oncology and Cancer Research. The insulin studies live in Diabetologia and Acta Diabetologica. Exercise oncology research is published in sports medicine journals across multiple fields; the same patients, different reading lists.
I no longer treat patients where I’m looking at glucose and insulin as just a pre-diabetic issue. It is a metabolic terrain issue.
Dr. Mary Ann Martin (Dr. Hormone Hacker)
Terrain - matters. The cancer research community has invested enormous resources in studying tumor genetics. But the environment in which those tumors grow? That’s been treated as secondary. Dr. Martin is arguing it’s primary.
“Where your metabolic health is, the environment that you’re creating in your body, is going to determine if you get cancer, how fast it spreads, whether you stay in remission.”
Research on stage III colon cancer patients found that those who ate a high-insulinogenic diet after diagnosis had nearly three times the risk of recurrence or death compared to those with the lowest insulinogenic diets. The hazard ratio was 2.77 for disease-free survival. This wasn’t about whether they had cancer - they all did. It was about whether their metabolic environment let it come back.
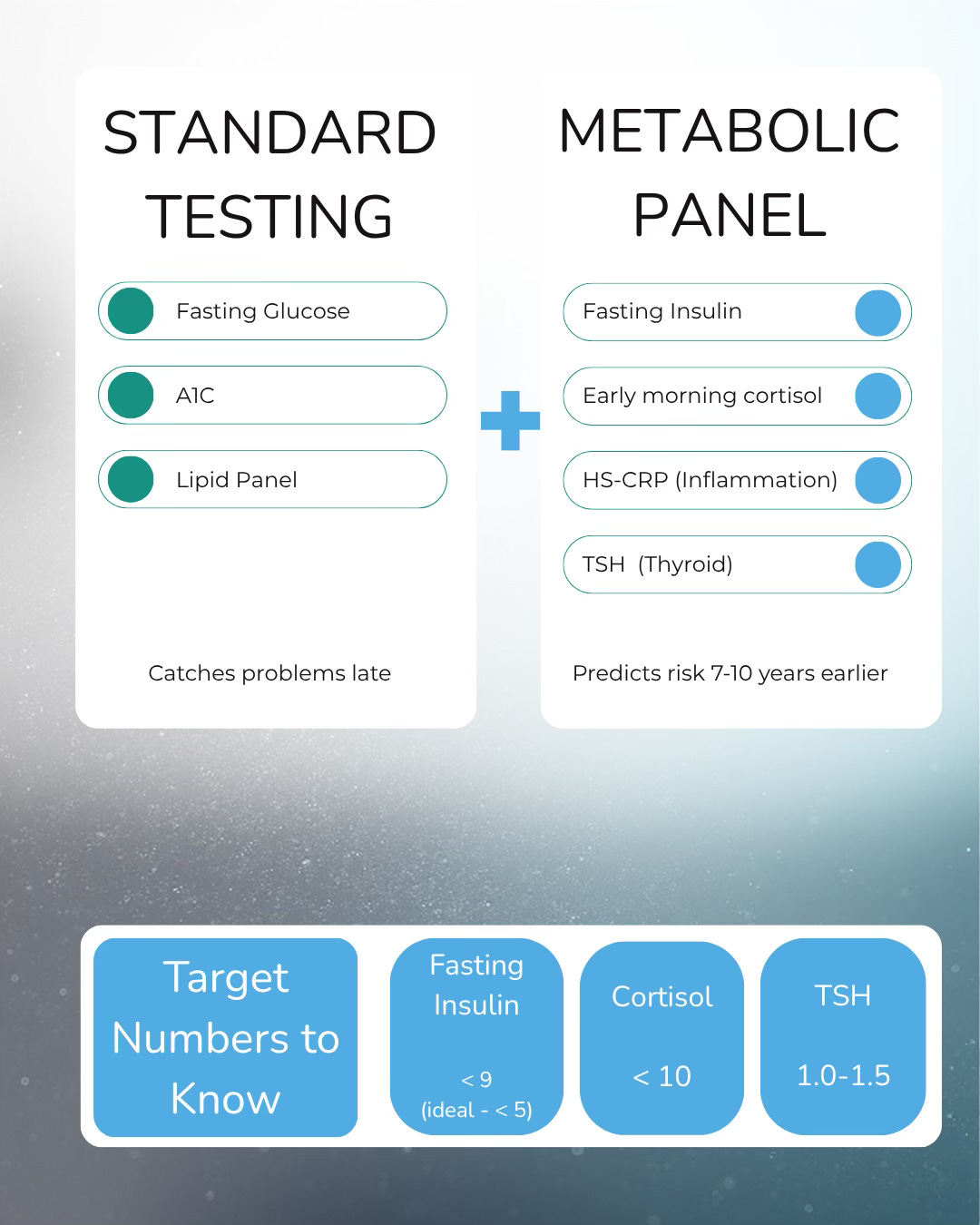
The Testing Gap
When Dr. Martin calls fasting insulin “the single most powerful predictor of early insulin resistance,” she’s making a clinical claim with research backing. The test predicts future diabetes seven to ten years before glucose or A1C show anything abnormal.
So why isn’t everyone getting it?
One hundred million Americans - one in three - are currently pre-diabetic. Eighty percent don’t know. Dr. Martin argues this is because we’re using the wrong diagnostic lens. The reference ranges on your lab report don’t reflect the levels at which damage occurs.
She recommends shooting for fasting insulin below 9, ideally below 5. If you’re above that, you’re in the range where insulin starts acting like a growth factor.
The Treatment Paradox
Here’s where it gets complicated for cancer patients: Treatment itself can spike your glucose and insulin. Steroids, commonly given to manage chemotherapy side effects, can raise blood sugar by 50 points or more. It can take four to six weeks after stopping steroids for levels to normalize. And a third of patients who require steroids go on to develop permanent diabetes.
Add to that the nausea management strategy of crackers, toast, and ginger ale - all high-glycemic foods that spike blood sugar rapidly. As Dr. Martin noted, “You’re putting all of these carbs... and all of this is contributing to that milieu that is helping the cancer to grow.”
The alternative isn’t suffering through nausea. It’s smarter choices: whole-grain toast instead of white, ginger tea instead of ginger ale, a cracker with fiber instead of a saltine. Small swaps that don’t change your carb intake but do change your insulin response.
What You Can Do
Before your next lab work, ask for fasting insulin, early morning cortisol (between 6-8 AM), and HS-CRP (highly sensitive C-reactive protein). If your doctor questions it, tell them you want a complete picture of your metabolic and inflammatory status.
Consider a continuous glucose monitor, even for 30 days. At $200 out of pocket, you’ll get data that guides you for years: which foods spike you, whether sleep affects your levels, what a post-meal walk actually does.
And recognize that bringing this research to your care team isn’t challenging their expertise - it’s bridging a gap between specialties that medical training created. You’re not the problem. You’re the solution.
Links:
Exercise and Cancer in Kicking Cancer’s Ass:
Medical References
1. Lancet Oncology 2010 - Pooled analysis of 17 prospective studies on IGF-1 and breast cancer Key TJ, et al. “Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies.” The Lancet Oncology, 11(6), 530-542.
Full text (PMC): https://pmc.ncbi.nlm.nih.gov/articles/PMC3113287/
PubMed: https://pubmed.ncbi.nlm.nih.gov/20472501/
2. Cancer Medicine 2020 - Mendelian randomization study on IGF-1 and colorectal cancer Larsson SC, et al. “Insulin-like growth factor-1 and site-specific cancers: A Mendelian randomization study.” Cancer Medicine, 9(18), 6836-6842.
Link: https://onlinelibrary.wiley.com/doi/10.1002/cam4.3345
3. NHANES Population Study - Hyperinsulinemia and cancer mortality in non-obese and obese people Tsujimoto T, et al. “Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study.” Cancer Medicine, 6(5), 1069-1078.
Full text (PMC): https://pmc.ncbi.nlm.nih.gov/articles/PMC5435954/
PubMed: https://pubmed.ncbi.nlm.nih.gov/28390156/
4. Cremona Study - Insulin resistance and cancer mortality at 15-year follow-up Perseghin G, et al. “Insulin resistance/hyperinsulinemia and cancer mortality: the Cremona study at the 15th year of follow-up.” Acta Diabetologica, 49(6), 421-428.
PubMed: https://pubmed.ncbi.nlm.nih.gov/22215126/
5. CALGB 89803 - Dietary insulin load and colon cancer recurrence Meyerhardt JA, et al. “Dietary Insulin Load and Cancer Recurrence and Survival in Patients With Stage III Colon Cancer: Findings From CALGB 89803 (Alliance).” Journal of the National Cancer Institute, 111(2), 170-179.